As an IVD (In Vitro Diagnostic) Korean manufacturer, accessing the EU (European Union) market can…
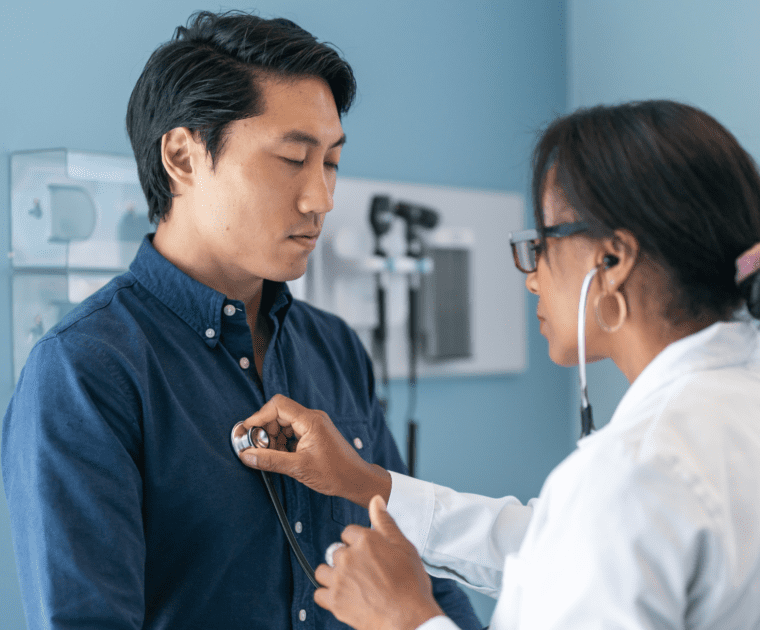

As an IVD (In Vitro Diagnostic) Korean manufacturer, accessing the EU (European Union) market can…

Most in vitro diagnostic medical devices require the involvement of a notified body or approved…

Introducing new restrictions for Butylated Hydroxytoluene (BHT) and Acid Yellow 3 in cosmetic products, the Commission Regulation (EU) 2022/2195 brings changes to the industry

MoCRA deadlines you need to keep in mind when selling cosmetic products in the U.S

Obelis Wins a Second Trends Gazelle Award.Growing from Medium-Sized to Big Enterprises CategoryWant to know more? Visit our page!

We are thrilled to announce that Obelis Group has been chosen as one of the regulatory consultants for the Luxemburgish Ministry of Economy’s Fit 4 Innovation

To prevent market disruptions and give manufacturers enough time to certify their medical devices under…

The EU proposal aims to introduce new hazard classes based on scientific criteria for endocrine disruptors as well as other chemicals which can accumulate in living organisms or enter the water cycle.

In addition to our Customer Success Department, Obelis has recently introduced a new client-focused Department: Customer Care. Read more here!

There was a general agreement between the Council of the EU representing the EU Member States and the European Commission to extend the transitional period (legacy period) to the Medical Devices Regulation (MDR). Read all the details in our article!