In December 2022, the U.S. passed the Modernisation of Cosmetics Regulation Act (MoCRA): the most extensive amendment to the US law on cosmetic products since 1938.
The law has several implications for manufacturers selling cosmetic products in the United States. Below is a summary of the important deadlines you should meet to ensure your cosmetics can be placed lawfully on the US market.
Immediate call to action for cosmetics manufacturers
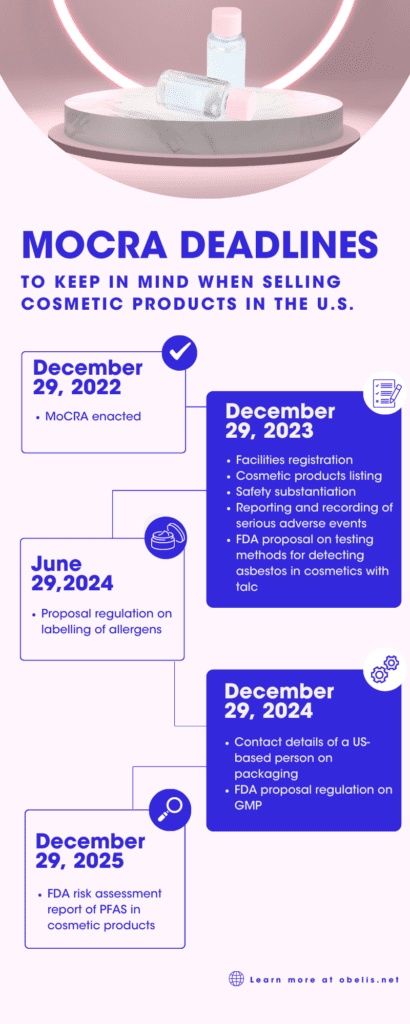
MoCRA will apply as of December 29, 2023, hence by that date, beauty brands will have to:
- Register their facilities with the FDA;
- List their cosmetic products with the FDA;
- Start communicating to the FDA the serious adverse events reported by consumers;
- Hold the documentation to substantiate the safety of their products.
Considering this, companies outside the United States must appoint a US Agent.
Labelling requirements
Regarding labelling, MoCRA establishes some new requirements. In particular,
- By June 29, 2024, the FDA must issue regulations on fragrance allergens to be labelled when they exceed certain concentrations. MoCRA directs the FDA to take account of the requirements on allergens already applicable in the EU.
- Not later than December 29, 2024, cosmetic packaging must bear the contact details of a US-based person to whom consumers can report serious adverse events.
FDA next steps
On top of new rules on the labelling of allergens, MoCRA sets some deadlines for the FDA to publish the following:
- A proposal on standardised testing methods for detecting asbestos (a silicate mineral carcinogen by inhalation) in cosmetic products containing talc by December 29, 2023. The final guidance shall be issued within 180 days from that date.
- A proposal regulation on Good Manufacturing Practices (GMP) by December 29, 2024, that will have to be finalised before December 29, 2025;
- A report on its risk assessment of PFAS (Per- and polyfluoroalkyl substances) in cosmetic products by December 29, 2025.
How can Obelis help you?
In the highly competitive world of cosmetic products, staying ahead of regulatory changes can be a real challenge. With MoCRA, ensuring your cosmetic products meet the new requirements is more important than ever.
At Obelis, we have the experience and expertise to help you navigate these changes and ensure your products are compliant. By appointing us as your US Agent or in-country regulatory consultant, you can focus on your core business while we handle the compliance process.
Don’t let regulatory changes hold your business back. Contact us today to learn how we can help you stay ahead of the game.
Francesca Santacatterina
Publications Department
04/05/2023
References:
- Authenticated U.S. Government Information. (2022). Modernisation of Cosmetics Regulations Act. Retrieved on 04/05/2023
- (2022). Talc. Retrieved on 04/05/2023