A definitive guide to CE marking in Europe. Discover what a CE marking is, what products require a CE mark, and why a CE mark is required on the EU market.


A definitive guide to CE marking in Europe. Discover what a CE marking is, what products require a CE mark, and why a CE mark is required on the EU market.

European Commission presented a proposal for a Regulation on cybersecurity requirements for products. Read more!

Discover the new GDPR obligations for businesses and organizations within the EU and UK!
Vigilance and Post-Market Surveillance definition, differences and their scope. Visit our webpage for all the details!

The European Commission issued a proposal to restrict microplastics. The regulation will have an impact on products containing those substances. Read more!
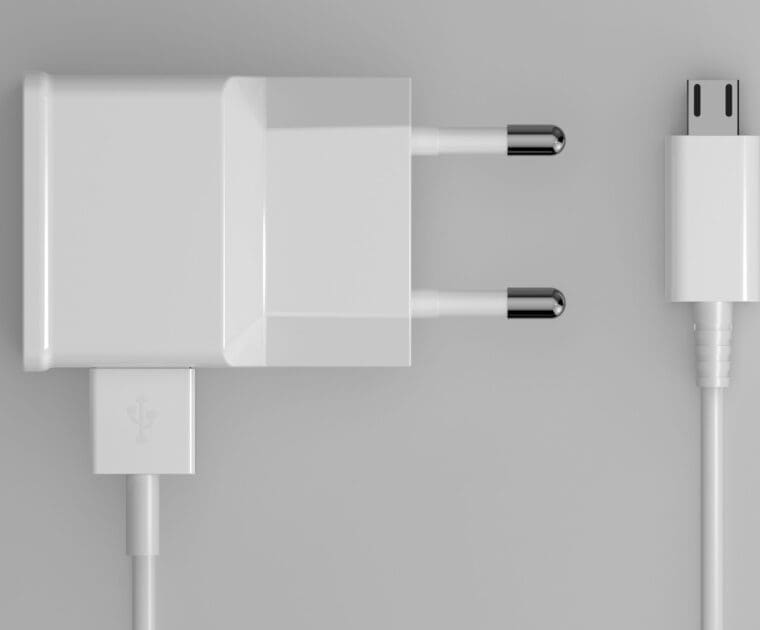
One more step is taken towards a common charger in the EU for all electronic devices. Learn more!

The European Commission's proposal for a new Batteries Regulation recommended several new elements that will effectively improve the quality of the batteries circulating in the single market. Read more!





The European Commission published a proposal to revise the CPR in order to address several shortcomings of the current legislation. Find out all the details!





The European Commission(EC) proposed a revision of the Radio Equipment Directive 2014/53/EU in order to introduce a common charger for electronic devices and reduce waste. Read more!





The European Union restricts hazardous substances in electrical and electronic equipment. Do you want to find out which substances? Visit our page!