The US government issued the Modernisation of Cosmetics Regulation Act (MoCRA) of 2022, which will apply as of December 29, 2023.


The US government issued the Modernisation of Cosmetics Regulation Act (MoCRA) of 2022, which will apply as of December 29, 2023.
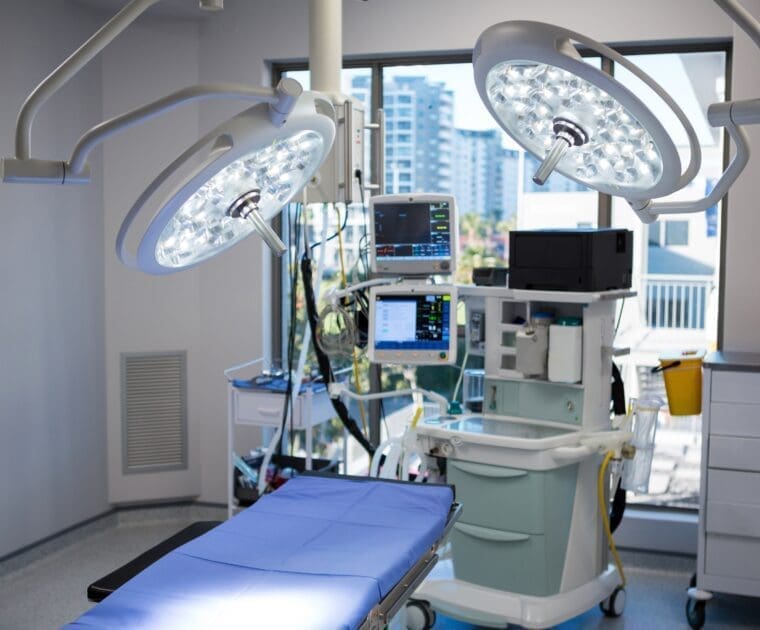
Medical Device and In-vitro Diagnostic Industry Discussion: Key Takeaways on IVDR and MDR Implementation Challenges

To prevent market disruptions and give manufacturers enough time to certify their medical devices under…

The new EU Regulation on in vitro diagnostic devices (IVDR), applicable since May 26, 2022, affected the classification and compliance process of COVID-19 tests.

The EU proposal aims to introduce new hazard classes based on scientific criteria for endocrine disruptors as well as other chemicals which can accumulate in living organisms or enter the water cycle.

In addition to our Customer Success Department, Obelis has recently introduced a new client-focused Department: Customer Care. Read more here!

An interview with Sarah You about her experience in following one of our expert’s professional trainings on EU Regulations and the insights she gained from it.

In November 2022, the Belgian government notified the EU of a draft Royal Decree to limit the placing on the market of single-use plastic products. Read all the details in our article!

There was a general agreement between the Council of the EU representing the EU Member States and the European Commission to extend the transitional period (legacy period) to the Medical Devices Regulation (MDR). Read all the details in our article!

Register and learn all the details about EUDAMED, the European database for medical devices and In-vitro diagnostics!!