Cosmetics are a part of our daily routine, but do we really pay attention to the safety information labelled on them?
The EU Cosmetics Regulation sets out mandatory labelling requirements for cosmetic products to communicate safety information to consumers. It is essential that manufacturers ensure their products’ labels include all necessary information before entering the EU market.
Two of the most commonly misunderstood labelling requirements are the expiry date and the Period After Opening (PAO).
What exactly do these labelling requirements mean, and how do you comply with them?
The expiry date and PAO indicate how long a cosmetic product, stored under appropriate conditions, keeps its function and can be used safely (minimum durability). The minimum durability is determined through a stability test (either accelerated or real-life study) and is taken into account by the safety assessor in the evaluation of the safety of the product.
Depending on the type and nature of the product, its minimum durability can vary. This can be relative to several factors:
- How the product is used or handled (interaction with human bodies, bacteria, fungi, etc.);
- Where and how the product is stored (temperature and exposure to light);
- Whether the product can dry out or become too moist (change its consistency) over time;
- Chemical make-up of the product and whether that may lead to a product separating into its various elements.
Expiry date and PAO — How to label it on the cosmetic packaging
According to the EU Cosmetics Regulation, both the inner and outer packaging of a cosmetic product must include the following:
- The expiry date if its minimum durability is less than 30 months; or
- The Period After Opening (PAO) if its minimum durability is more than 30 months.
EU authorities confirmed that a product cannot display both an expiry date and PAO.
Expiry date and PAO can be indicated using text that must be translated into the national languages of the EU countries where the product is marketed. Otherwise, beauty brands can use the symbols from the EU Cosmetics Regulation: the hourglass for the expiry date and the open jar for the PAO.
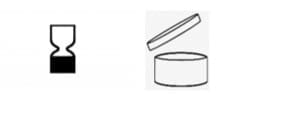
Next to the hourglass, the manufacturer should add the expiry date (ex., 31/12/2024). Inside the jar, an indication of the months the product can be safely used after opening is added (ex., 12 M).
Exceptions — Products that do not need a PAO
Due to their characteristics, the Period After Opening is irrelevant for certain products. Therefore, if a product’s minimum durability is over 30 months, no indications shall be added to the labels. Some examples:
- Single-use products;
- Products that do not come into contact with the external environment due to the type of container (ex., aerosol or airless dispensers);
- Products with low microbiological risk.
At Obelis, we offer a wide range of services for cosmetics brands that want to sell their products on the EU market, including laboratory testing and labelling review. Rely on our team of regulatory professionals to ensure your products comply with the EU requirements: we make the compliance process as smooth as possible.
Contact us today to know how we can help you.
Author: Davide Turchi | Regulatory Affairs Department
Co-Author: Francesca Santacatterina | Publications
Updated on May 2023


