Introducing new restrictions for Butylated Hydroxytoluene (BHT) and Acid Yellow 3 in cosmetic products, the Commission Regulation (EU) 2022/2195 brings changes to the industry


Introducing new restrictions for Butylated Hydroxytoluene (BHT) and Acid Yellow 3 in cosmetic products, the Commission Regulation (EU) 2022/2195 brings changes to the industry

Discover the new GDPR obligations for businesses and organizations within the EU and UK!

Discover the new GDPR obligations for businesses and organizations within the EU and UK!

On 23 May 2023 the Official Journal of the EU published a new law, the General Product Safety Regulation (GPSR). From 13 December 2024, manufacturers will have to comply with the new GPSR.

MoCRA deadlines you need to keep in mind when selling cosmetic products in the U.S

Obelis Wins a Second Trends Gazelle Award.Growing from Medium-Sized to Big Enterprises CategoryWant to know more? Visit our page!

Manufacturers! Interested in the European Commission proposal for legacy devices? Our consultant will be offering an interpretation of the European Commission proposal for legacy devices regarding the following date: March 20, 2023

A definitive guide to CE marking in Europe. Discover what a CE marking is, what products require a CE mark, and why a CE mark is required on the EU market.
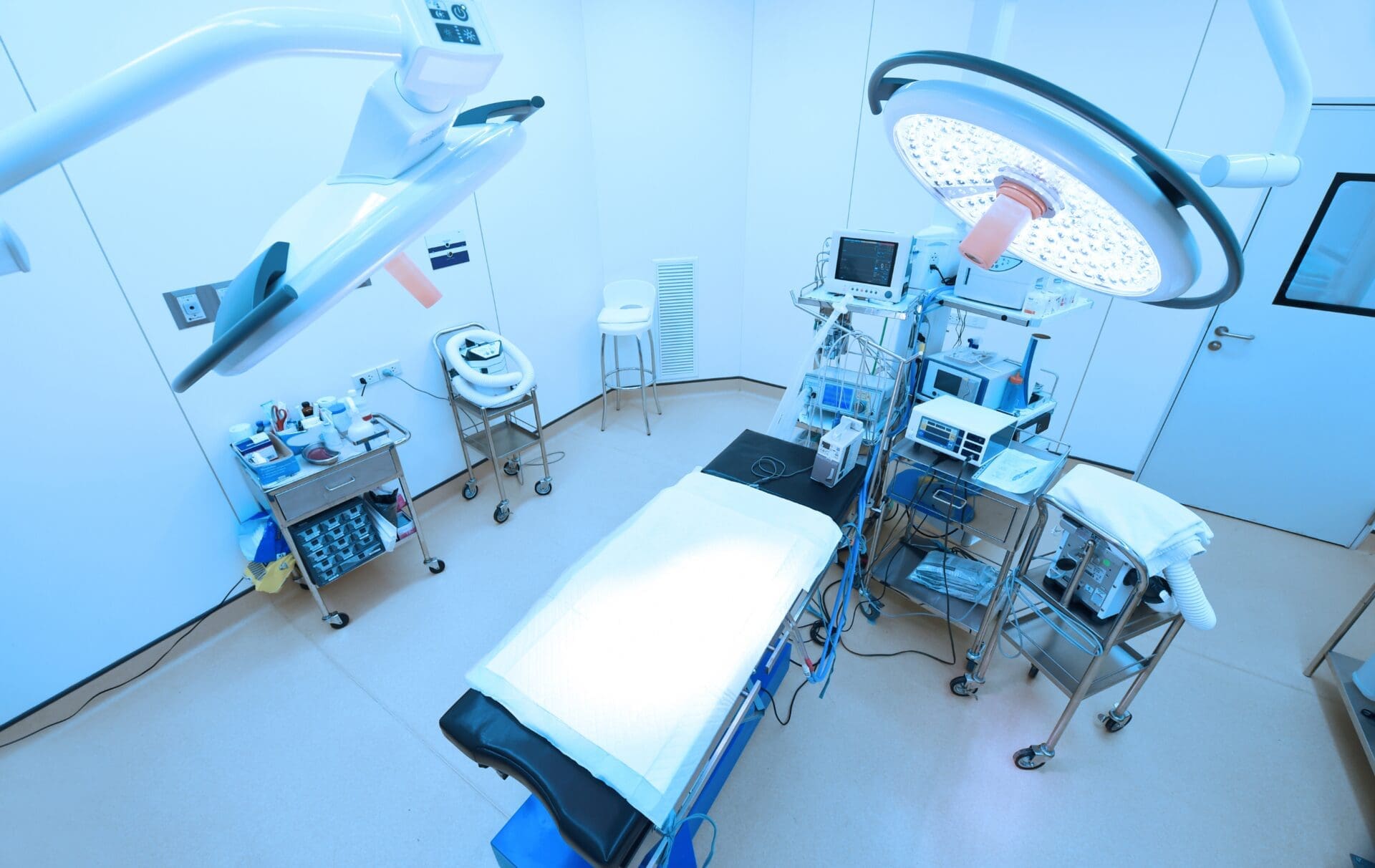
Complying with the rules on distance selling of medical devices and in vitro diagnostics device is a fundamental step when a manufacturer chooses to sell the devices online.

We are thrilled to announce that Obelis Group has been chosen as one of the regulatory consultants for the Luxemburgish Ministry of Economy’s Fit 4 Innovation