The year 2026 brings numerous regulatory deadlines affecting cosmetic product labelling across several territories, including the European Union, United Kingdom, Switzerland, Canada, and the United States.


The year 2026 brings numerous regulatory deadlines affecting cosmetic product labelling across several territories, including the European Union, United Kingdom, Switzerland, Canada, and the United States.
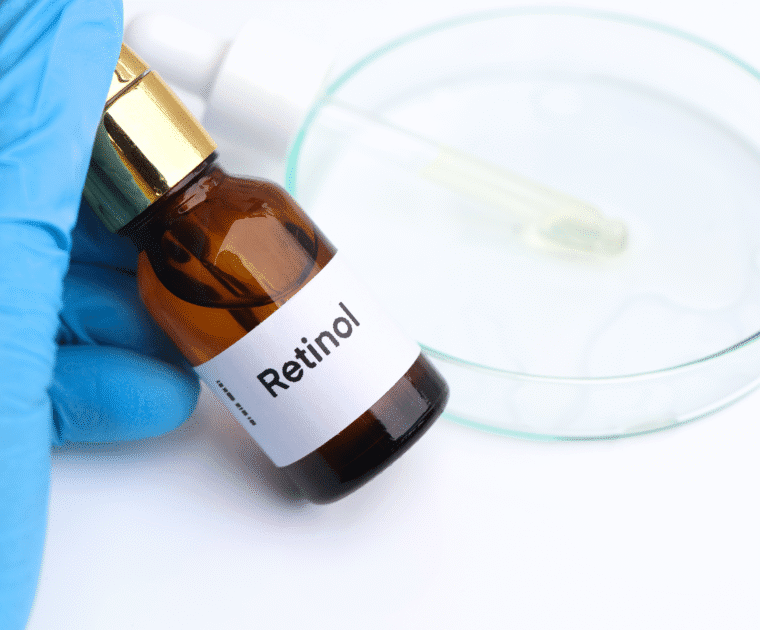
The European Commission has announced new cosmetics compliance deadlines affecting Retinol, Triclosan, and several nanomaterials. From late 2025, products containing these ingredients must meet stricter safety limits or be withdrawn from the EU market. Learn what these changes mean for your formulations and labelling.

Australia, often referred to as the skin cancer capital of the world, is facing a…

In 2025, the EU introduced Omnibus Acts VII and VIII, banning and restricting several substances in cosmetics due to CMR classifications. These changes require immediate compliance. Learn how Obelis can help ensure your products meet the latest regulations.

From 1 July 2025, the EU will enforce new limits on Homosalate in cosmetics, allowing a max of 7.34% in face products. Reformulation may be required to ensure compliance.

The European Union represents one of the world’s most valuable and influential cosmetics markets, valued…

In June 2022, Quebec passed Bill 96, An Act respecting French, the official and common language of Québec, which amended the Charter of the French Language. These amendments significantly affect labelling and advertising requirements for businesses, including those in the cosmetics industry.

From May 2025, the EU bans 3-(4′-methylbenzylidene)-camphor in cosmetics. Learn what this means for product compliance and how beauty brands should prepare.

Discover Health Canada’s update requiring a Canadian-based representative for foreign cosmetic brands and how Obelis can help with compliance.

Key February 2025 alert for the cosmetics industry: new EU rules restrict ingredients like Genistein, Kojic Acid, and Alpha-Arbutin under Annex III and ban specific nanomaterials under Annex II.